Continual Clean, Powered By:
UV-C AND OZONE
Our patented UVZone® technology harnesses the dual power of UVC and ozone, eliminating up to 99.9993% of pathogens in seconds—providing a continual clean from the ground up.
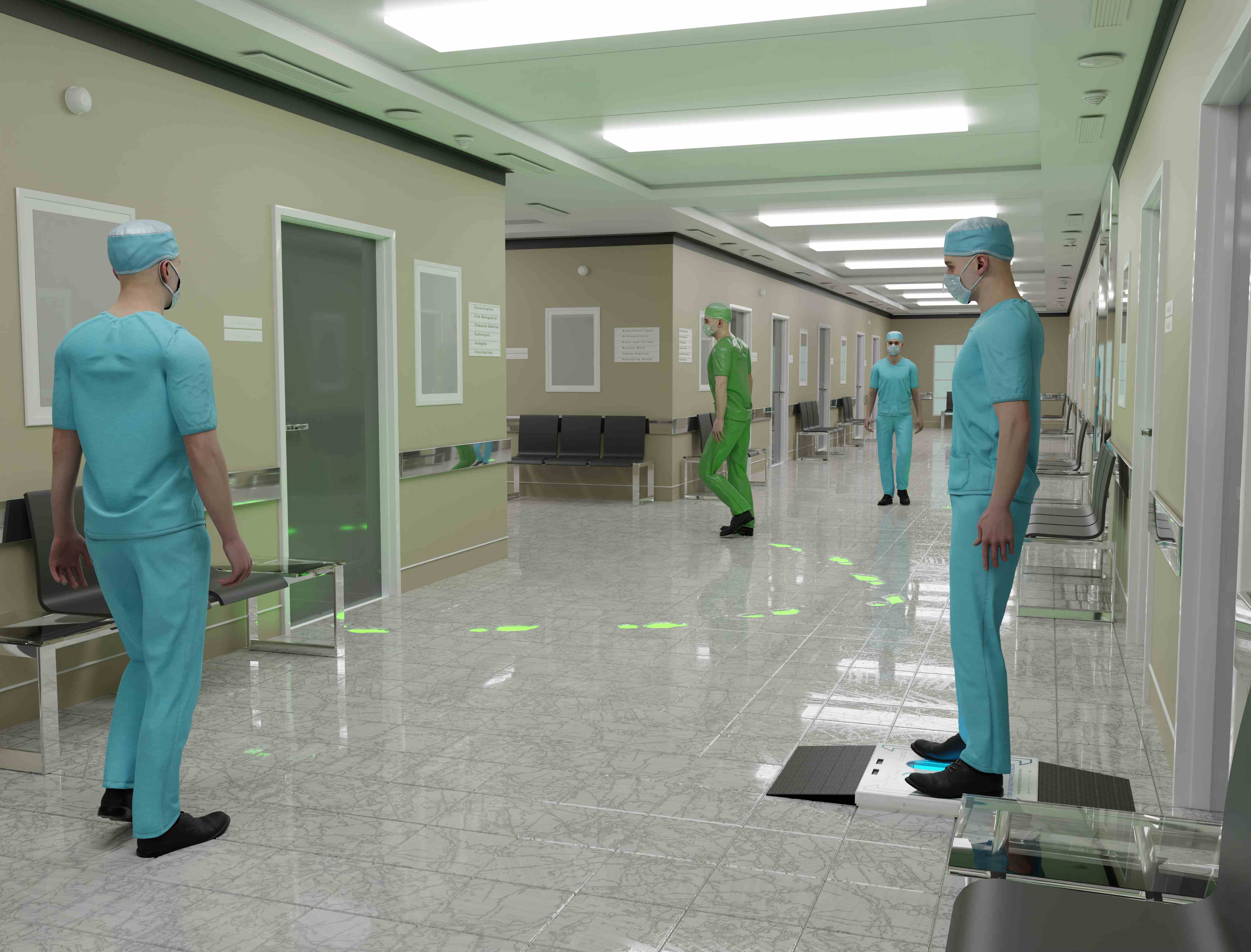
UVZone® Technology
Industry-leading pathogen mitigation combining UV-C light and ozone for superior protection in 6-10 seconds

Dual-Action Technology Explained
UV-C light and ozone work together to provide superior pathogen reduction on shoe soles
UV-C Light at 254nm
Germicidal UV-C light at 254 nanometers penetrates microbial cell walls and damages nucleic acids (DNA/RNA), forming thymine dimers that prevent replication. This renders pathogens unable to reproduce or cause infection.
Ozone Generation
UV-C light at 185nm converts oxygen (O₂) into ozone (O₃), a powerful oxidizing agent. Ozone molecules penetrate crevices and porous materials that light cannot reach directly, oxidizing proteins and lipids in microbial cell membranes.
Synergistic Disinfection
The combination of UV-C and ozone provides more comprehensive pathogen reduction than either method alone
Surface Treatment
UV-C light directly disinfects exposed surfaces of the shoe sole
Deep Penetration
Ozone gas reaches into treads, grooves, and porous materials
Comprehensive Reduction
Combined action achieves superior pathogen reduction rates

UVZone® Technology
Industry-leading pathogen mitigation combining UV-C light and ozone for superior protection in 6-10 seconds

Product Specifications
Engineered for maximum efficacy and ease of use
Dimensions & Operation
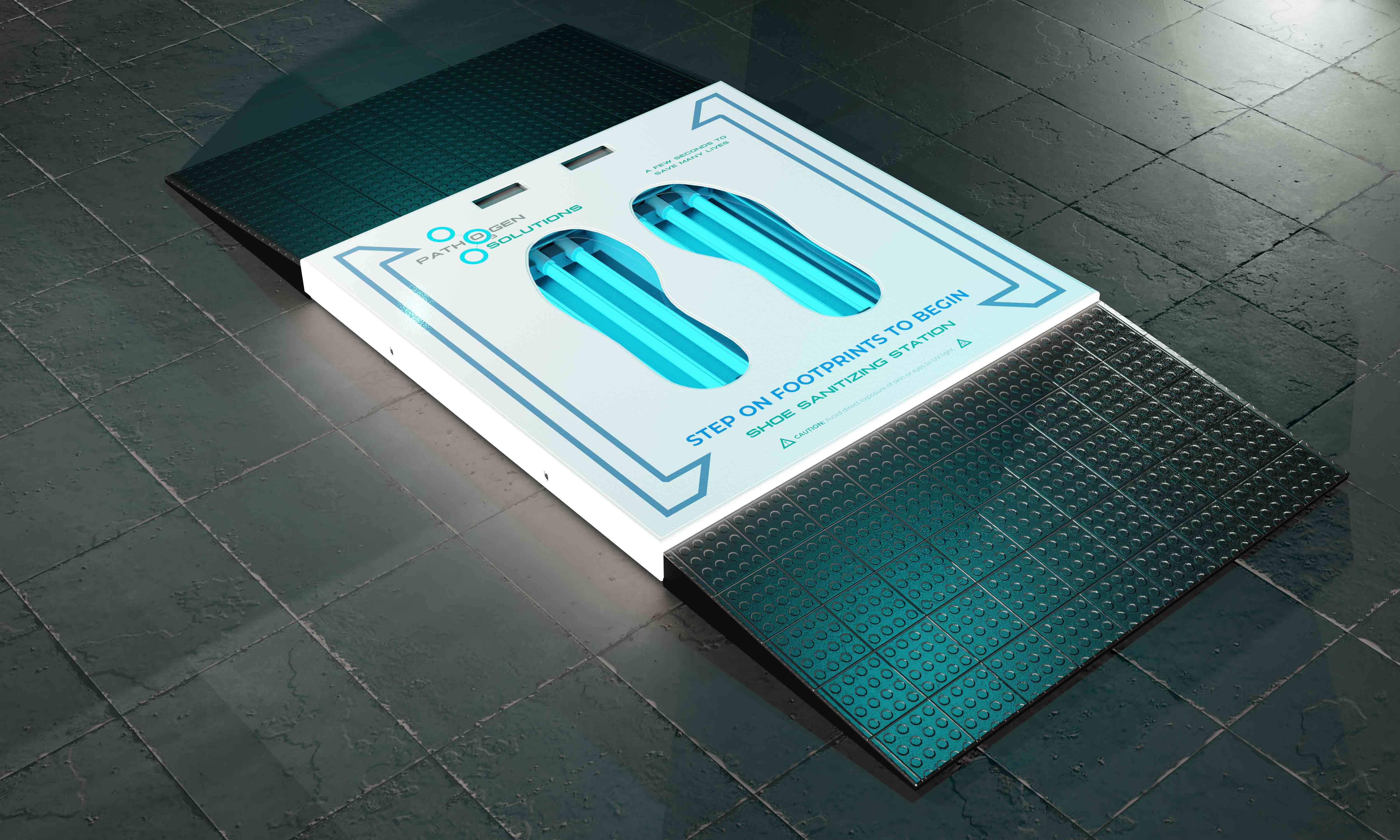
Plug & Play Installation
Simply plug into any standard outlet and place at entry points. No special installation or maintenance required.
Key Highlights
Controls On-Site Traffic
Creates biosafety perimeter support around critical areas
Encourages Compliance
Simple step-on activation ensures consistent use
Industry-Leading Microbial Reduction
Up to 99.9993% elimination in 6-10 seconds
Fast Treatment
6-10 second cycles for high-traffic areas
Dry, mess-free
No liquid chemicals, no-mess, no additional staff hours required
For Use With Any Footwear
Boots, shoes, sneakers—all materials
Dual-Action Technology Explained
Explore how UV-C and ozone work together to achieve industry-leading pathogen elimination
Synergistic Disinfection
The combination of UV-C and ozone creates a comprehensive disinfection that eliminates pathogens in seconds—achieving results more effective than either alone.
Ozone Weakens Cell
Ozone oxidizes and weakens cell membranes and viral capsids
UV-C Penetrates
UV-C light penetrates weakened cells and inactivates genetic material
Total Inactivation
Pathogens are rendered inactive and unable to replicate


The Hidden Threat: Hospital Floors
Cleveland VA Medical Center study reveals how pathogens spread from contaminated floors throughout healthcare facilities
Study Methodology
Inoculation
30×30cm floor area adjacent to patient bed inoculated with bacteriophage MS2 (1×10⁸ PFU/mL)
Monitoring
Daily sampling of patient hands, footwear, high-touch surfaces, adjacent rooms, and nursing stations
Rapid Spread
Virus detected throughout facility within 24 hours via footwear, hands, and contaminated objects
How Pathogens Spread From Floors
Footwear Contact
100% of patient footwear tested positive for the virus within 24 hours, with high concentrations (3.4-4.0 log₁₀ PFU)
Hand Transfer
40-62.5% of patient hands contaminated through touching footwear, bed linens, or objects in contact with floor
Surface Contamination
High-touch surfaces (bed rails, call buttons, phones) contaminated at rates of 58-76%, especially within 3 feet of bed
Facility-Wide Spread
Healthcare workers unknowingly carry pathogens on shoes and hands to adjacent rooms, nursing stations, and throughout facility
Portable Equipment
Wheelchairs, medication carts, and vital signs equipment become vectors, spreading contamination between patient areas
Persistent Contamination
Virus persisted on floors for 3+ days with only 1-2 log reduction, maintaining transmission potential
The UVZone® Solution
By eliminating 99.9993% of pathogens from footwear in 6-10 seconds, UVZone® stations break the contamination chain at its source—preventing floor-to-facility spread before it begins.
Scientific Validation
Independently tested and verified by leading laboratories. Our efficacy claims are backed by rigorous scientific studies.
NSF International Tested
Third-party laboratory validation of efficacy claims
TÜV SÜD Certified
International safety and quality certification
EPA Registered
Registered with the Environmental Protection Agency
ISO 9001:2015
Manufactured in ISO-certified facility in the USA
NSF International Testing
Comprehensive efficacy testing against 8 healthcare-associated pathogens. All organisms showed >99.9% reduction at 8-10 seconds.
Pathogens Tested:
Norovirus Study
Testing against Murine Norovirus as a surrogate for human norovirus. Achieved >4.31 log reduction at 6, 8, and 10 seconds, leaving zero plaque.
Pathogens Tested:
Foodborne Pathogen Study
Custom device study based on modified ASTM E1153 testing foodborne pathogens on glass carriers simulating shoe soles.
Pathogens Tested:
Avian Influenza Study
Inactivation of Three Subtypes of Influenza A Virus by a Commercial Device Using Ultraviolet Light and Ozone
Pathogens Tested:
Need More Information?
Contact us to receive full study reports, technical specifications, and application guidance for your specific use case.
Contact Our TeamCertifications & Compliance
Rigorously tested and certified for safety and efficacy
Environmental Protection Agency registered device
International certification for electrical safety and performance
Produced in ISO 9001:2015 certified facility in the USA
Experience UVZone® Technology
See the dual-action UV-C and ozone system in action at your facility